
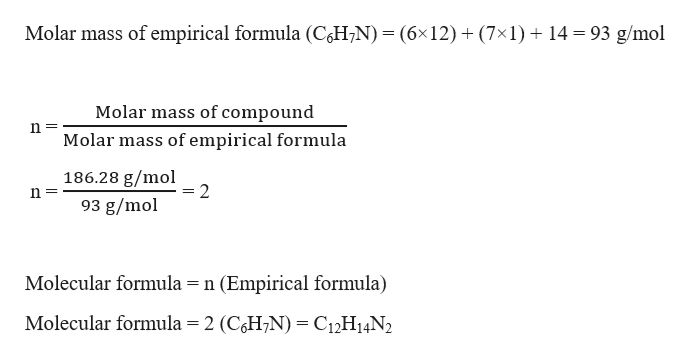
The mole ratios we found in the previous step are 1, 2 and 1, all of which are whole numbers. The penultimate step in the procedure is converting the mole ratios to whole numbers, in case they aren’t already. Number of moles of Z/3.33 = 3.33/3.33 = 1 Step 4: Convert mole ratios into whole numbers We can find the mole ratios of each element by dividing the individual mole values by the smallest mole value found. From the previous step, we see that element X and Z both have 3.33 moles in the compound, whereas Y has 6.66 moles. Now, look for the element with the least number of moles in the compound. Step 3: Calculate mole ratios of each element The number of moles are as follows: moles of X are 40/12.o1 = 3.33 mol moles of Y are 6.7/1.00784 = 6.6 mol and the moles of Z are 53.3/15.999 = 3.33 mol. ( Note: One can find the molar mass of any element by performing a simple Google search.)
Number of moles = Amount of the element present (in grams) / Molar mass of the elementĬoming back to our sample compound… the molar mass of X is 12.0107 g/mol, Y is 1.00784 g/mol and Z is 15.999 g/mol. The formula to find the number of moles of an element from its amount is: One mole of an element means that about 6.022×10 23 atoms of the element are present. In case you’ve been living under a rock, moles are a unit of measurement used to measure the quantities of small particles, such as atoms, molecules, electrons, protons, neutrons, etc. In Step 2, we find the number of moles of each element using the mass values found in the previous step. Step 2: Convert the amount of each element to moles
If the amount of each element is already available in grams, this step doesn’t need to be performed. We only need to perform this step when the composition of the compound is provided in percentage form.
If we have 100 grams of the sample compound, that would imply that X is 40 g, Y is 6.7 g and Z is 53.3 g. Let’s say that the results of a chemical analysis reveal that the compound X aY bZ c has 40.0% of X, 6.7% of Y, and 53.3% of Z. We convert these percentage values into simple numbers by assuming that we have 100g of the sample compound. Chemical analysis of the compound X aY bZ c yields information regarding the amount of each element (X, Y and Z) in percentage form. Let’s assume that the compound whose empirical formula is to be found is X aY bZ c. We start the procedure by finding the exact amount (in grams) of each element that makes up the compound being studied. Empirical formula calculation explained through a flowchart Step 1: Find the mass (amount) of each element in the compound


 0 kommentar(er)
0 kommentar(er)
